1) Veldeman L, Madani I, Hulstaert F, De Meerleer G, Mareel M, De Neve W. Evidence behind use of intensity-modulated radiotherapy:a systematic review of comparative clinical studies. Lancet Oncol. 2008;9:367-375.


4) Zhang B, Mo Z, Du W, Wang Y, Liu L, Wei Y. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma:A systematic review and meta-analysis. Oral Oncol. 2015;51:1041-1046.


5) Marta GN, Silva V, de Andrade Carvalho H, de Arruda FF, Hanna SA, Gadia R, et al. Intensity-modulated radiation therapy for head and neck cancer:systematic review and meta-analysis. Radiother Oncol. 2014;110:9-15.


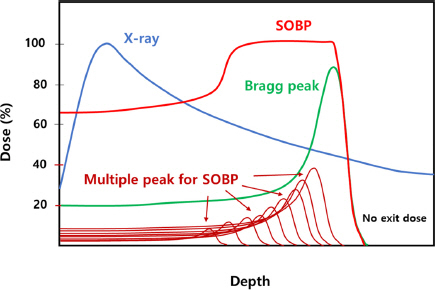
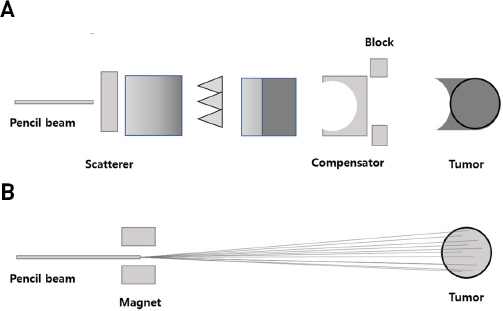
6) Rong Y, Welsh J. Basics of particle therapy II biologic and dosimetric aspects of clinical hadron therapy. Am J Clin Oncol. 2010;33:646-649.


7) Palm A, Johansson KA. A review of the impact of photon and proton external beam radiotherapy treatment modalities on the dose distribution in field and out-of-field;implications for the long-term morbidity of cancer survivors. Acta Oncol. 2007;46:462-473.


9) Alterio D, Marvaso G, Ferrari A, Volpe S, Orecchia R, Jereczek-Fossa BA. Modern radiotherapy for head and neck cancer. Semin Oncol. 2019;46:233-245.


10) Moreno AC, Frank SJ, Garden AS, Rosenthal DI, Fuller CD, Gunn GB, et al. Intensity modulated proton therapy (IMPT) - The future of IMRT for head and neck cancer. Oral Oncol. 2019;88:66-74.


12) Leeman JE, Romesser PB, Zhou Y, McBride S, Riaz N, Sherman E, et al. Proton therapy for head and neck cancer:expanding the therapeutic window. Lancet Oncol. 2017;18:e254-e265.


13) Li X, Lee A, Cohen MA, Sherman EJ, Lee NY. Past, present and future of proton therapy for head and neck cancer. Oral Oncol. 2020;110:104879.


14) Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Goitein M, Loeffler JS, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407-421.


15) Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419-472.

17) Willers H, Allen A, Grosshans D, McMahon SJ, von Neubeck C, Wiese C, et al. Toward A variable RBE for proton beam therapy. Radiother Oncol. 2018;128:68-75.


18) Mohan R, Grosshans D. Proton therapy - Present and future. Adv Drug Deliv Rev. 2017;109:26-44.


20) Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175:57-63.

21) Weber DC, Rutz HP, Pedroni ES, Bolsi A, Timmermann B, Verwey J, et al. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base:the Paul Scherrer Institut experience. Int J Radiat Oncol Biol Phys. 2005;63:401-409.


25) Patel SH, Wang Z, Wong WW, Murad MH, Buckey CR, Mohammed K, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases:a systematic review and meta-analysis. Lancet Oncol. 2014;15:1027-1038.


27) Pasalic D, Ludmir EB, Allen PK, Thaker NG, Chapman BV, Hanna EY, et al. Patient-reported outcomes, physician-reported toxicities, and treatment outcomes in a modern cohort of patients with sinonasal cancer treated using proton beam therapy. Radiother Oncol. 2020;148:258-266.


28) Svajdova M, Dubinsky P, Kazda T. Radical external beam re-irradiation in the treatment of recurrent head and neck cancer:Critical review. Head Neck. 2021;43:354-366.


29) Lee J, Shin IS, Kim WC, Yoon WS, Koom WS, Rim CH. Reirradiation with intensity-modulated radiation therapy for recurrent or secondary head and neck cancer:Meta-analysis and systematic review. Head Neck. 2020;42:2473-2485.


30) Foster CC, Fan M, Lee NY, Yom SS, Heaton CM, Deraniyagala R, et al. Is It Worth It?Consequences of Definitive Head and Neck Reirradiation. Semin Radiat Oncol. 2020;30:212-217.


32) Phan J, Sio TT, Nguyen TP, Takiar V, Gunn GB, Garden AS, et al. Reirradiation of Head and Neck Cancers With Proton Therapy:Outcomes and Analyses. Int J Radiat Oncol Biol Phys. 2016;96:30-41.


33) McDonald MW, Zolali-Meybodi O, Lehnert SJ, Estabrook NC, Liu Y, Cohen-Gadol AA, et al. Reirradiation of Recurrent and Second Primary Head and Neck Cancer With Proton Therapy. Int J Radiat Oncol Biol Phys. 2016;96:808-819.


34) Verma V, Rwigema JM, Malyapa RS, Regine WF, Simone CB. Systematic assessment of clinical outcomes and toxicities of proton radiotherapy for reirradiation. Radiother Oncol. 2017;125:21-30.


37) Slater JD, Yonemoto LT, Mantik DW, Bush DA, Preston W, Grove RI, et al. Proton radiation for treatment of cancer of the oropharynx:early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys. 2005;62:494-500.


45) Moon SH, Cho KH, Lee CG, Keum KC, Kim YS, Wu HG, et al. IMRT vs. 2D-radiotherapy or 3D-conformal radiotherapy of nasopharyngeal carcinoma :Survival outcome in a Korean multi-institutional retrospective study (KROG 11-06). Strahlenther Onkol. 2016;192:377-385.
46) Lewis GD, Holliday EB, Kocak-Uzel E, Hernandez M, Garden AS, Rosenthal DI, et al. Intensity-modulated proton therapy for nasopharyngeal carcinoma:Decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck. 2016;38:E1886-1895.


47) Liu SW, Li JM, Chang JY, Yu JM, Chen Q, Jiang QA, et al. A treatment planning comparison between proton beam therapy and intensity-modulated x-ray therapy for recurrent nasopharyngeal carcinoma. J Xray Sci Technol. 2010;18:443-450.


48) Minatogawa H, Yasuda K, Dekura Y, Takao S, Matsuura T, Yoshimura T, et al. Potential benefits of adaptive intensity- modulated proton therapy in nasopharyngeal carcinomas. J Appl Clin Med Phys. 2021;22:174-183.


49) Holliday EB, Garden AS, Rosenthal DI, Fuller CD, Morrison WH, Gunn GB, et al. Proton Therapy Reduces Treatment- Related Toxicities for Patients with Nasopharyngeal Cancer:A Case-Match Control Study of Intensity-Modulated Proton Therapy and Intensity-Modulated Photon Therapy. International Journal of Particle Therapy. 2015;2:19-28.

50) Alterio D, D'Ippolito E, Vischioni B, Fossati P, Gandini S, Bonora M, et al. Mixed-beam approach in locally advanced nasopharyngeal carcinoma:IMRT followed by proton therapy boost versus IMRT-only. Evaluation of toxicity and efficacy. Acta Oncol. 2020;59:541-548.
53) Richard P, Sandison G, Dang Q, Johnson B, Wong T, Parvathaneni U. Dental amalgam artifact:Adverse impact on tumor visualization and proton beam treatment planning in oral and oropharyngeal cancers. Pract Radiat Oncol. 2015;5:e583-588.


54) Saager M, Peschke P, Brons S, Debus J, Karger CP. Determination of the proton RBE in the rat spinal cord:Is there an increase towards the end of the spread-out Bragg peak? Radiother Oncol. 2018;128:115-120.


55) Sanchez-Parcerisa D, Lopez-Aguirre M, Dolcet Llerena A, Udias JM. MultiRBE:Treatment planning for protons with selective radiobiological effectiveness. Med Phys. 2019;46:4276-4284.


57) Kozak KR, Smith BL, Adams J, Kornmehl E, Katz A, Gadd M, et al. Accelerated partial-breast irradiation using proton beams:initial clinical experience. Int J Radiat Oncol Biol Phys. 2006;66:691-698.


58) Hansen CR, Friborg J, Jensen K, Samsoe E, Johnsen L, Zukauskaite R, et al. NTCP model validation method for DAHANCA patient selection of protons versus photons in head and neck cancer radiotherapy. Acta Oncol. 2019;58:1410-1415.


59) Rwigema JM, Langendijk JA, Paul van der Laan H, Lukens JN, Swisher-McClure SD, Lin A. A Model-Based Approach to Predict Short-Term Toxicity Benefits With Proton Therapy for Oropharyngeal Cancer. Int J Radiat Oncol Biol Phys. 2019;104:553-562.


60) Brodin NP, Kabarriti R, Pankuch M, Schechter CB, Gondi V, Kalnicki S, et al. A Quantitative Clinical Decision-Support Strategy Identifying Which Patients With Oropharyngeal Head and Neck Cancer May Benefit the Most From Proton Radiation Therapy. Int J Radiat Oncol Biol Phys. 2019;104:540-552.


61) Zhang YY, Huo WL, Goldberg SI, Slater JM, Adams JA, Deng XW, et al. Brain-Specific Relative Biological Effectiveness of Protons Based on Long-term Outcome of Patients With Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys. 2021.

62) Apinorasethkul O, Kirk M, Teo K, Swisher-McClure S, Lukens JN, Lin A. Pencil beam scanning proton therapy vs rotational arc radiation therapy:A treatment planning comparison for postoperative oropharyngeal cancer. Med Dosim. 2017;42:7-11.